
Remember your biology teacher’s beloved amoeba? They are members of a vast, biodiverse group of single-cell organisms: the protozoa. Biologists have described some 80,000 species of protozoa. Many more undoubtedly await discovery.1
Although unicellular, protozoa are neither simple nor inconsequential. Some protozoa are more complex than human cells and several cause dire social, economic and health problems.1 For instance, Toxoplasma gondii and the various species of Plasmodium, which both belong to a subgroup of protozoa called the Apicomplexa, cause toxoplasmosis and malaria respectively.1
During 2021, there were about 247 million cases of malaria and 619,000 deaths from the parasite globally.2 Almost half the world’s population is at risk of contracting malaria when a female mosquito feeds on their blood. Mosquitoes transmit Plasmodium in their saliva.2,3
Between the 16th and 19th centuries, ague, the British term for malaria, was common across the fens and marshes of southern England.3,4 Today, we import malaria: on average, 1425 malaria cases annually. Between 2012 and 2021 imported malaria killed, on average, six people a year.5 However, these figures probably underestimate the number of imported cases.3 Nurses should remind travellers to parts of the world where malaria is endemic to pack mosquito nets, insect repellents and anti-malarial drugs.
Unlike malaria, T. gondii is common in the UK. A study from Scotland, for example, reported that 13.2% of 3273 blood donors (2006-2009) had antibodies to T. gondii, suggesting that they had been infected at some point. Six of the 184 patients who gave blood in all four years became seronegative.6 So, the lifetime risk of T. gondii may be more greater than these figures suggest. Indeed, DNA samples from 151 human brains (2008–2012) suggested a prevalence of T. gondii of 17.9%.6
Most people do not realise they have been infected: usually, T. gondii is asymptomatic.7 But T. gondii can cause mortality and serious health problems if the parasite infects an immunodeficient patient or pregnant woman.6-8 Indeed, the legacy of maternal infections can last the rest of her child’s life. Chronic T.gondii infections increase the risk of various neurological and psychiatric conditions.9
Introducing T. gondii
In 1908, the French scientists Charles Nicolle and Louis Manceaux discovered T. gondii in the gundi (Ctenodactylus gondi), a cute, chubby rodent from North Africa.7,10,11 Nicolle and Manceaux proposed the name Toxoplasma to reflect the curved shape of the parasite’s infectious stage. Toxon is the Greek for bow. Plasm is a late-Latin word for mould that biologists use to denote life. Gondii may be a misspelling of gundi.10 At about the same time, the Italian scientist Alfonso Splendore found T.gondii in rabbits while working in Brazil.7,11
T. gondii reproduces sexually only in cats, the definitive host.12,13 Female and male gametes combine in the epithelial cells of the cat’s small intestine. This forms oocysts, which cats excrete in faeces.13 Oocytes are remarkably resilient, surviving in water for long periods and for more than a year in some environmental conditions.8,13
Over the next few days, infectious sporozoites develop inside the oocysts.14 This means that the parasite is not infectious for 1–5 days after the cat excretes the oocysts in faeces.15 But warm-blooded animals often ingest oocysts laden with sporozoites. Once ingested, sporozoites leave the oocyte, invade the small intestine and covert into tachyzoites.14 In turn, tachyzoites spread throughout the body and rapidly multiply.14,16
In animals other than cats, T. gondii reproduces asexually.12,13 Numerous mammals and birds harbour T. gondii including red deer, wild boar, voles, mice, shrews and Californian sea-otters.1,7,17,18 Cats, of course, kill small rodents and birds, and feed on cadavers, which is how the parasite reaches the definitive host (figure 1).12,13 Some of the six subtypes (genotypes) of T. gondii only infect wild animals.16
T. gondii may infect humans who ingest food and water contaminated with oocysts or raw or undercooked meat that contains cysts.17 T. gondii can form cysts in the brain, retina, muscle and liver cells.12,13,16 Cysts contain hundreds of bradyzoites, another stage in development, which slowly multiply.13 Nevertheless, even a few cysts in food can cause toxoplasmosis. This means that some people eating infected meat will miss a cyst, while others at the same meal develop toxoplasmosis.19
Meat from pigs and sheep are more likely to include tissue cysts than cuts from cattle.16 In the 1960s, for example, a Parisian hospital fed people raw meat as part of a treatment for tuberculosis.10 The proportion of patients who developed T. gondii antibodies rose from 10% a year on admission to 50% annually when the hospital offered barely seared beef and horse. When the hospital switched to lamb chops the proportion who seroconverted increased to 100%.10
Soil harbouring T. gondii can mean vegetables transmit the parasite.19 Table 1 summarises some of the precautions people can take to reduce the risk of infection from food. Blood transfusion and organ transplants can also, in rare cases, spread T. gondii.13,20
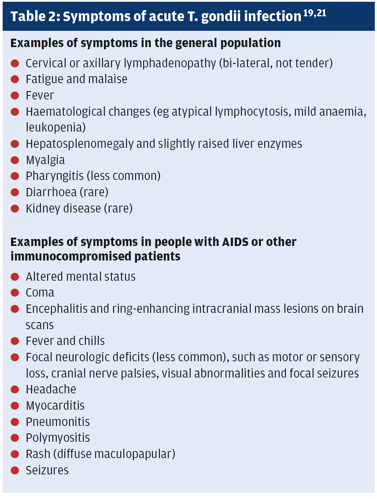
Most T. gondii infections do not cause symptoms.7 However, after an incubation period of, usually, 7–30 days between 10% and 20% of people acutely infected by T. gondii develop bilateral, non-tender cervical or axillary lymphadenopathy.19,21 A few people also experience mild flu-like symptoms (table 2).21 These symptoms, which are not age-specific, can persist for weeks.19,21
Symptoms caused by T. gondii infections are almost always mild and self-limiting.19,21 Nevertheless, T. gondii can mimic diseases such as influenza, Lyme disease, Q fever, haematological changes and mumps.19 Pharyngitis caused by T. gondii can mimic infectious mononucleosis.21 The mild symptoms mean toxoplasmosis is easy to miss and diagnosis often delayed.19
In addition, without treatment (see the British National Formulary: bnf.nice.org.uk/treatment-summaries/antiprotozoal-drugs), persistent cysts in nerves and other cells in the central nervous system that change levels of brain neurotransmitters.12,20 This may be one reason why chronic T. gondii infections seem to increase the risk of several neurological and psychiatric disorders.9
Such changes in the brain also help explain why rodents infected with T. gondii show increased activity, less fear of new stimuli and decreased vigilance for cats and other predators.12 Rodents and chimpanzees infected with T. gondii do not show their usual innate aversion to the urine of feline predators.22,23
A recent study found that deer with T. gondii antibodies were culled earlier than those negative for the parasite in areas with high levels of human activity. Uninfected deer in areas with high human activity tend to be shy to avoid predators’ attention. Seropositive deer seem to have become more risk-prone.24 T. gondii is one of several parasites that trigger physiological, reproductive, behavioural and other changes that increase the ability to pass their genes on to their offspring (geneticists call this fitness) at the expense of their hosts.22
Maternal toxoplasmosis
In 1937, researchers discovered that T. gondii can cross the placenta and damage the fetus.7 About 80% of pregnant women acutely infected with T. gondii are asymptomatic.8 So, prevention is essential (table 1).

The proportion of surviving fetuses with toxoplasmosis increases from 15% to 30% to 60% in the first, second and third trimesters respectively.21 The risk of T. gondii transmission increases to 25–50% and 50–70% in the second and third trimesters respectively.8
The consequences can be devastating. Maternal T. gondii increases the risk of miscarriage or stillbirth.8,13 Some children severely infected in utero die within a few days of birth.13 A fetus with a T. gondii infection can develop, for example:8,13,21
- Hepatosplenomegaly (enlarged liver and spleen)
- Hydrocephalus (fluid accumulates in the brain
- Jaundice
- Microcephaly (small head circumference and brain damage)
- Neurological and psychomotor impairment
- Rash
- Retinochoroiditis (also called chorioretinitis; discussed later)
Retinochoroiditis, learning difficulties, mental retardation, seizures and other severe problems can emerge months or years later as the child grows.8,13,21
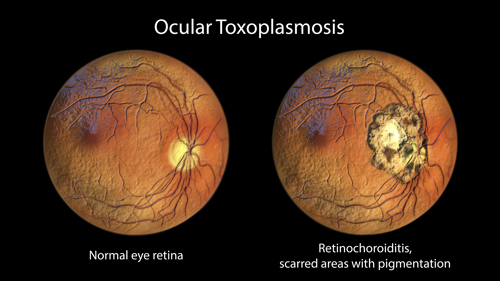
Ocular toxoplasmosis often arises from congenital infection that typically reactivates during the patients’ teens and 20s. In rare cases, ocular toxoplasmosis develops after acute infections. Focal necrotising retinitis (inflammation of the retina) and inflammation of the choroid (middle layer in the wall of the eye between the sclera, the whites, and the retina) cause ocular pain, blurred vision, and, sometimes, blindness. Relapses are common.21 The subtype (strain) of T. gondii may influence the risk of developing eye disease.19 Infection outside the eye and CNS and mainly affects immunocompromised patients (table 2).21
T. gondii may not attract the same attention as malaria or the other diseases in humans and domesticated animals caused by parasitic protozoa, such as Trypanosoma cruzi (Chagas’ disease) and species of Leishmania (Leishmaniasis) and Cryptosporidium (cryptosporidiosis), that can devastate parts of the economically developing world.1 But T. gondii is much more common in the UK and nurses can help limit the risk that pregnant women and immunocompromised patients will be infected. While T. gondii may not be in the global spotlight, the effects of this common parasite can still last a lifetime.
Mark Greener is a freelance medical writer
References
1. Goater TM, Goater CP and Esch GW, Parasitism: The Diversity and Ecology of Animal Parasites. Second ed 2014, Cambridge: Cambridge University Press.
2. World Health Organisation. Malaria. Available at www.who.int/news-room/fact-sheets/detail/malaria. Accessed November 2023.
3. Greener M. Defeating malaria: new weapons against one of our deadliest foes. Prescriber 2020;31:18-22.
4. Dobson M. “Marsh fever” – The geography of malaria in England. J Hist Geogr 1980;6:357-389.
5. UK Government. Malaria imported into the UK: 2021. Available at www.gov.uk/government/publications/malaria-in-the-uk-annual-report/malaria-imported-into-the-uk-2021. Accessed November 2023.
6. Burrells A, Opsteegh M, Pollock KG et al. The prevalence and genotypic analysis of Toxoplasma gondii from individuals in Scotland, 2006-2012. Parasit Vectors 2016;9:324.
7. Cox FE. History of human parasitic diseases. Infect Dis Clin North Am 2004;18:171-88.
8. Markovic-Denic L, Stopic M, Bobic B et al. Factors associated with Toxoplasma gondii seroprevalence in pregnant women: a cross-sectional study in Belgrade, Serbia. Pathogens 2023;12:
9. Greener M. Parasites and mental health. Prog Neurol Psychiatry 2022;26:8-10.
10. Ferguson DJ. Toxoplasma gondii: 1908-2008, homage to Nicolle, Manceaux and Splendore. Mem Inst Oswaldo Cruz 2009;104:133-48.
11. Ajioka JW and Morrissette NS. A century of Toxoplasma research. Int J Parasitol 2009;39:859-60.
12. Markovitz AA, Simanek AM, Yolken RH et al. Toxoplasma gondii and anxiety disorders in a community-based sample. Brain Behav Immun 2015;43:192-7.
13. Yu C-P, Chen B-C, Chou Y-C et al. The epidemiology of patients with toxoplasmosis and its associated risk factors in Taiwan during the 2007–2020 period. PLOS One 2023;18:e0290769.
14. Poukchanski A, Fritz HM, Tonkin ML et al. Toxoplasma gondii sporozoites invade host cells using two novel paralogues of RON2 and AMA1. PLOS ONE 2013;8:e70637.
15. Communicable Disease Center. Toxoplasmosis: General FAQs. Available at www.cdc.gov/parasites/toxoplasmosis/gen_info/faqs.html. Accessed November 2023
16. Attias M, Teixeira DE, Benchimol M et al. The life-cycle of Toxoplasma gondii reviewed using animations. Parasit Vectors 2020;13:588.
17. Abrantes AC and Vieira-Pinto M. 15 years overview of European zoonotic surveys in wild boar and red deer: A systematic review. One Health 2023;16:100519.
18. Kalmár Z, Sándor AD, Balea A et al. Toxoplasma gondii in small mammals in Romania: the influence of host, season and sampling location. BMC Vet Res 2023;19:177.
19. Dubey JP. Outbreaks of clinical toxoplasmosis in humans: five decades of personal experience, perspectives and lessons learned. Parasit Vectors 2021;14:263.
20. Rahdar M, Farbod Y, Seydinejad S et al. The effect of chronic experimental toxoplasmosis on some brain neurotransmitters level and behavior changes. Exp Parasitol 2023;251:108575.
21. Marie C and Petri W. Toxoplasmosis. MSD Manual (Professional version). Available at www.msdmanuals.com/en-gb/professional/infectious-diseases/extraintestinal-protozoa/toxoplasmosis. Accessed November 2023. 2022;
22. Fayard M, Dechaume-Moncharmont FX, Wattier R et al. Magnitude and direction of parasite-induced phenotypic alterations: a meta-analysis in acanthocephalans. Biol Rev Camb Philos Soc 2020;95:1233-1251.
23. Tsukahara T. Lions eat chimpanzees: The first evidence of predation by lions on wild chimpanzees. Am J Primatol 1993;29:1-11.
24. Nava M, Corlatti L, Formenti N et al. Parasite-mediated manipulation? Toxoplasma gondii infection increases risk behaviour towards culling in red deer. Biol Lett 2023;19:20230292.
