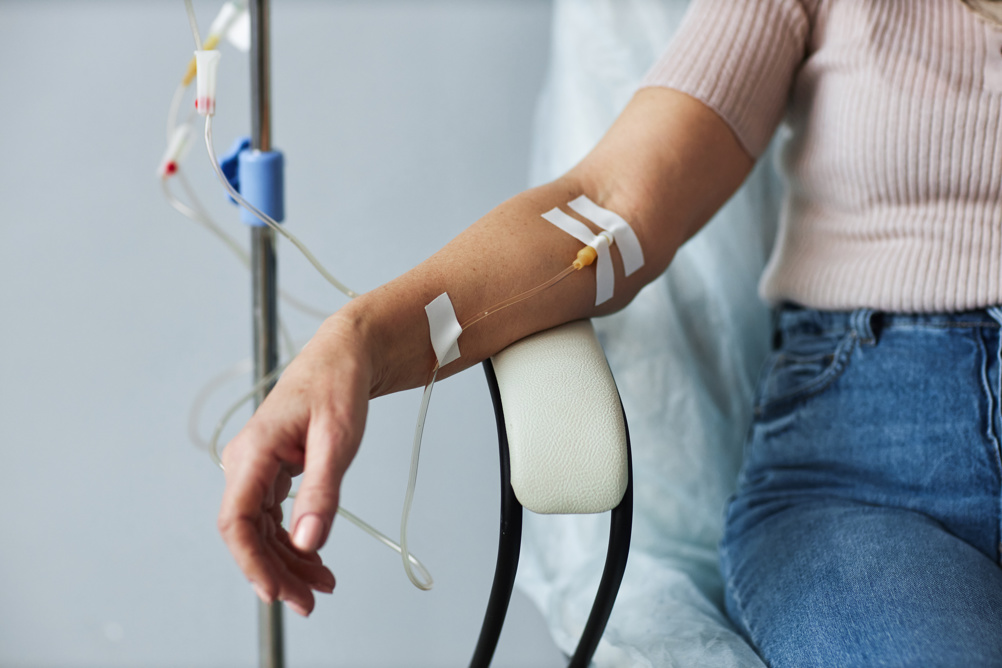
NHS England will be the first health service worldwide to introduce a pioneering ‘trojan horse’ therapy for blood cancer patients. The therapy was developed by GSK UK, who found in clinical trials that the treatment could potentially slow disease progression by nearly three times longer than existing treatments. The treatment works by entering cancer cells and releasing lethal molecules that destroy them from within. Following NICE approval, around 1,500 patients each year with multiple myeloma will now have access to the ‘antibody-drug conjugate’ belantamab mafodotin.
- New five-minute cancer jab could treat 15 cancers
- Government on a ‘mission to drive down waiting times’ as 80,000 receive faster cancer diagnosis
- Immunotherapy: the final cure for cancer?
‘We are delighted to have worked with NHS England and NICE on this significant recommendation in helping support unmet need in multiple myeloma,’ said Antoine Herbaux, Vice President and Head of Oncology UK, GSK. ‘Today’s announcement highlights an example of local innovation - Belantamab mafodotin was partly discovered in Stevenage, the first patient to receive it in clinical trials was in London, and now the UK is the first country to grant patient access. This milestone is a great example of the power of scientific innovation and open collaboration to achieve positive outcomes for patients in the UK’
This new treatment will be offered to eligible patients whose cancer has progressed or not responded to first-line treatment. Multiple myeloma is an incurable type of bone marrow cancer that can affect various bones including the spine and skull, with treatments aiming to halt the progression. Eligible patients will receive infusions every three weeks, in combination with other cancer drugs as well as regular eye assessments to monitor potential side effects.
‘Myeloma is an aggressive type of blood cancer, but we have seen steady improvements in patient outcomes thanks to new targeted therapies,’ said Professor Peter Johnson, NHS England’s National Clinical Director for Cancer. ‘I am delighted that patients in England will be the first to benefit from this treatment, which could keep cancer at bay for years longer, giving people more precious time with their loved ones. This therapy could be life-changing for many, and it is vital the NHS continues to secure rapid access to innovative treatments at affordable prices.’
